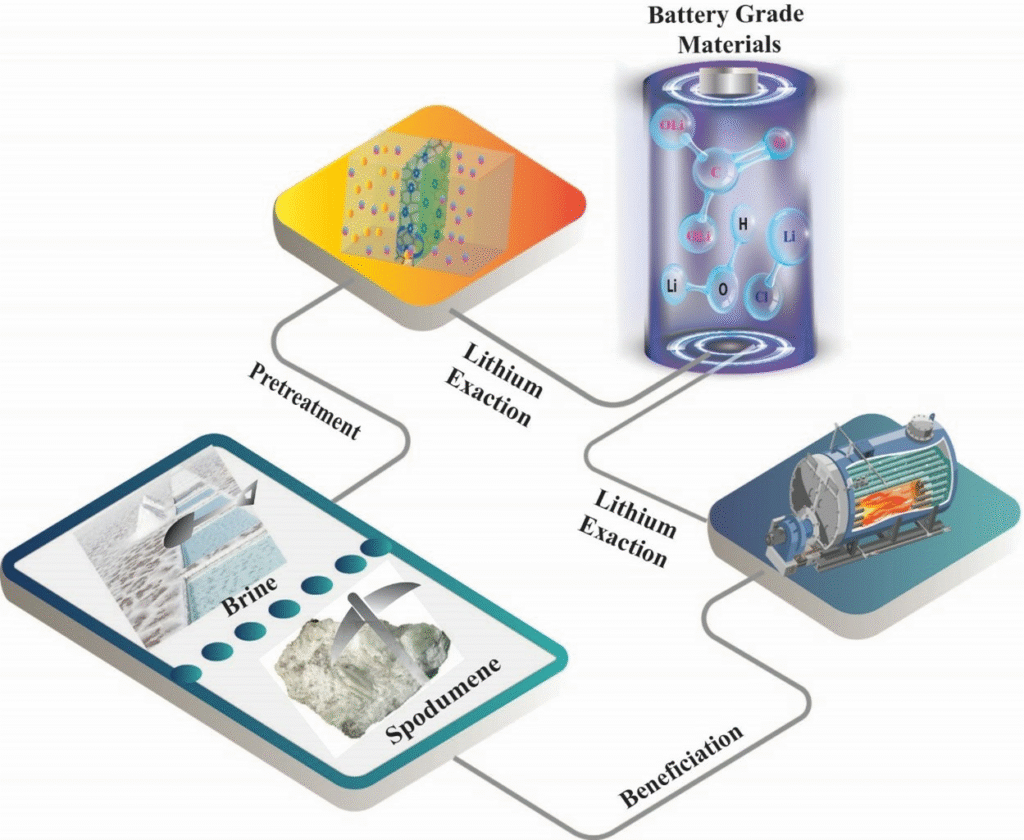
Lithium Mineral Extraction

Lithium mineral extraction refers to the process of recovering lithium from naturally occurring lithium-bearing minerals, primarily found in hard rock pegmatites, brines, and clay deposits. As demand for lithium surges due to its use in lithium-ion batteries, understanding the extraction methods and their economic and environmental implications is essential.
Here’s a comprehensive guide to how lithium is extracted from minerals and brines.
1. Types of Lithium Deposits
There are three primary sources of lithium:
| Deposit Type | Description |
|---|---|
| Hard Rock (Pegmatite) | Lithium is found in minerals like spodumene, lepidolite, and petalite in granite-rich environments. |
| Brine Deposits | Lithium is dissolved in salt-rich water in salt flats (salars) and closed basins. |
| Clay Deposits | Lithium is adsorbed onto clay minerals and extracted through chemical processing. |
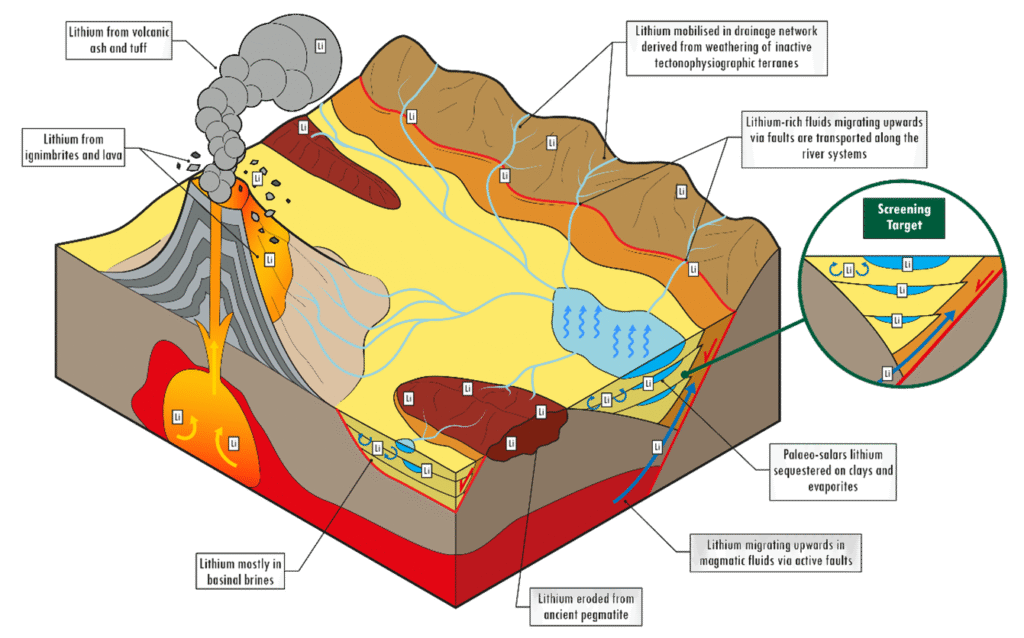
2. Hard Rock Lithium Extraction (Spodumene Mining)
Hard rock lithium extraction involves mining and processing lithium-rich minerals, especially spodumene, which is the most economically viable source.
Steps in Hard Rock Lithium Extraction:
- Mining: Open-pit or underground mining of lithium-bearing pegmatites.
- Crushing and Milling: Ore is crushed and ground into fine particles.
- Concentration: Froth flotation or magnetic separation is used to concentrate spodumene.
- Thermal and Chemical Processing:
- The spodumene is roasted at high temperatures (calcined).
- Then treated with sulfuric acid to produce lithium sulfate.
- Conversion to Battery-Grade Lithium:
- Lithium sulfate is further processed into lithium carbonate or lithium hydroxide.
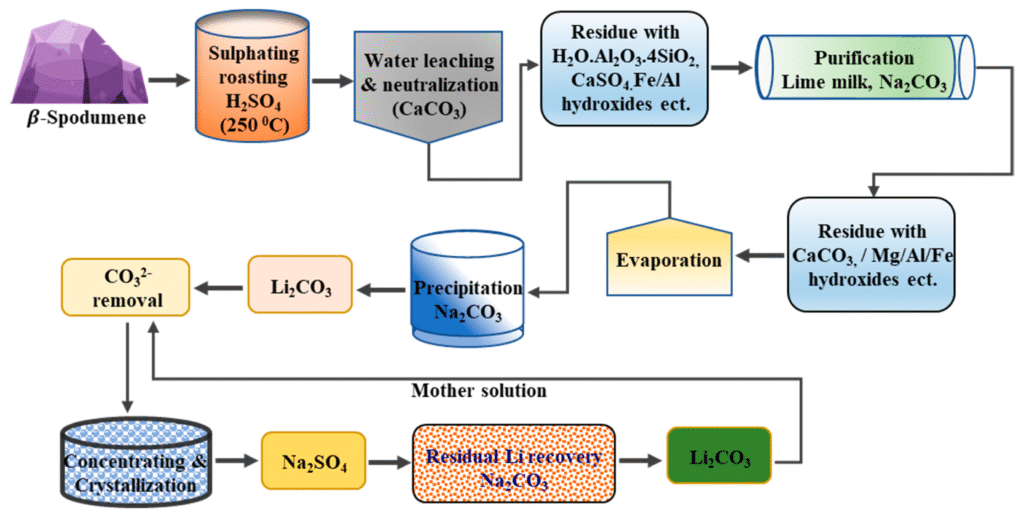
3. Lithium Brine Extraction
Brine extraction is the most cost-effective method and is used in regions like Chile, Argentina, and Bolivia (the “Lithium Triangle”).
Steps in Brine Lithium Extraction:
- Pumping Brine: Lithium-rich brine is pumped from underground reservoirs.
- Solar Evaporation: Brine is placed in large evaporation ponds for 12–18 months, allowing water to evaporate and concentrate lithium.
- Purification and Precipitation: Impurities are removed, and lithium is precipitated as lithium carbonate or converted to lithium hydroxide.
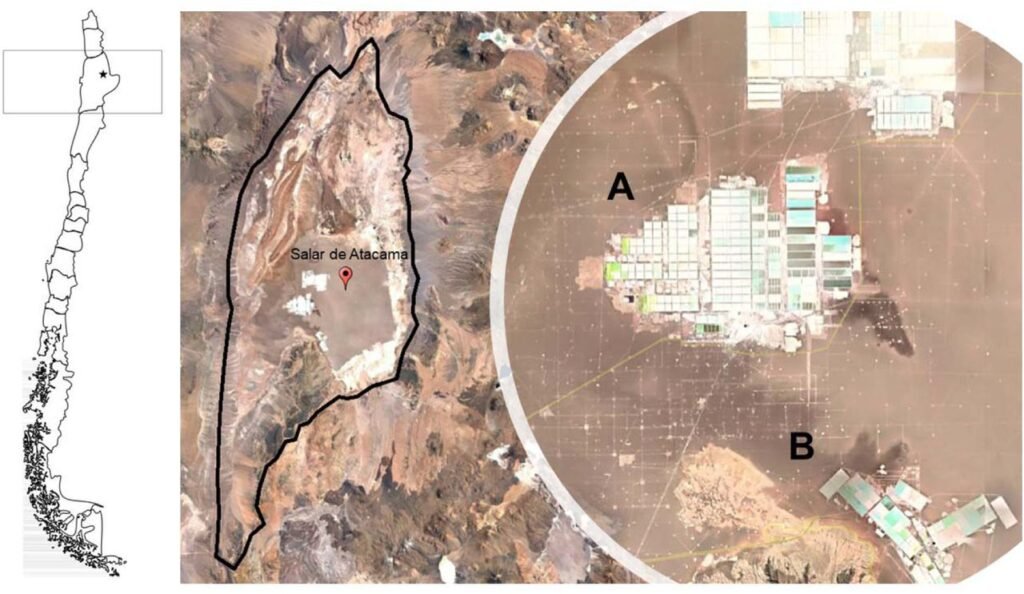
4. Lithium Extraction from Clay Deposits
Clay deposits are an emerging source of lithium, particularly in Nevada (USA), Serbia, and China.
Steps in Clay Lithium Extraction:
- Mining: Lithium-rich clay is excavated from open-pit mines.
- Leaching or Direct Extraction:
- Clay is treated with acid or other solvents to extract lithium ions.
- Some newer technologies use direct extraction without acid.
- Purification and Conversion: Lithium is purified and converted into carbonate or hydroxide.
5. Comparison of Extraction Methods
| Method | Source | Extraction Time | Cost | Water Use | Environmental Impact |
|---|---|---|---|---|---|
| Hard Rock Mining | Spodumene, lepidolite | 6–12 months | High | Moderate | Land disruption, waste rock |
| Brine Extraction | Salt flats (salars) | 12–18 months | Low | High | Water depletion in arid areas |
| Clay Extraction | Hectorite, smectite | 6–12 months | Medium | Moderate | Less water-intensive than brines |
6. Lithium Extraction and the Battery Supply Chain
Lithium extracted from minerals is used to produce lithium carbonate and lithium hydroxide, both of which are essential for EV battery production.
- Lithium Carbonate (Li₂CO₃): Used in LFP (lithium iron phosphate) batteries.
- Lithium Hydroxide (LiOH): Preferred for NCM and NCA cathode chemistries in high-energy-density EV batteries.
7. Environmental and Ethical Considerations
Lithium extraction raises several environmental and ethical concerns, especially in sensitive ecosystems.
Key Issues:
- Water usage in arid regions (e.g., Chile’s Atacama Desert)
- Soil and water contamination from acid leaching in hard rock and clay processing
- Community displacement and land use conflicts
- Carbon footprint of mining and chemical processing
Sustainability Efforts:
- Direct Lithium Extraction (DLE) for faster, more efficient brine processing
- Closed-loop water systems to reduce water consumption
- Battery recycling to recover lithium from used devices and EVs
8. Top Countries in Lithium Extraction
| Country | Main Lithium Source | Annual Production (2024–2025) |
|---|---|---|
| Australia | Hard rock (spodumene) | ~60,000 metric tons (LCE) |
| Chile | Brine | ~30,000 metric tons (LCE) |
| Argentina | Brine | ~10,000 metric tons (LCE) |
| China | Brine, clay, hard rock | ~20,000 metric tons (LCE) |
| United States | Clay, brine | ~5,000 metric tons (LCE) |
FAQs
Q1: How is lithium extracted from minerals?
A1: Lithium is extracted from hard rock minerals like spodumene through crushing, flotation, roasting, and acid leaching to produce lithium carbonate or hydroxide.
Q2: Is lithium extraction harmful to the environment?
A2: It can be, depending on the method. Brine extraction uses large amounts of water, while hard rock mining generates waste rock and tailings.
Q3: What is Direct Lithium Extraction (DLE)?
A3: DLE is an emerging technology that allows faster and more sustainable lithium recovery from brines using adsorption or membrane filtration instead of evaporation ponds.
Conclusion
Lithium mineral extraction is a vital process that supports the electric vehicle revolution, renewable energy storage, and modern electronics industry. While extraction methods vary—hard rock mining, brine evaporation, and clay leaching—each has its economic, environmental, and technological trade-offs.
As the world moves toward a clean energy future, developing sustainable extraction methods, recycling infrastructure, and diversified supply chains will be essential for responsible lithium production.

