Lithium Fluoride Mineral

Lithium fluoride (LiF) is a naturally occurring inorganic compound and can be classified as a mineral in rare geological settings. It is a simple ionic compound composed of lithium (Li⁺) and fluoride (F⁻) ions. While not as commonly known as lithium-bearing minerals like spodumene or lepidolite, lithium fluoride plays a role in industrial applications and is found in trace amounts in certain pegmatites and hydrothermal deposits.
1. What Is Lithium Fluoride (LiF)?
Lithium fluoride is a white, crystalline solid with high melting and boiling points. It is the least soluble alkali metal fluoride in water and has a high thermal and chemical stability, making it valuable in industrial and scientific contexts.
- Chemical formula: LiF
- Crystal structure: Cubic (similar to sodium chloride)
- Melting point: ~845°C
- Hardness: 3.5 on the Mohs scale
- Solubility: Very low in water
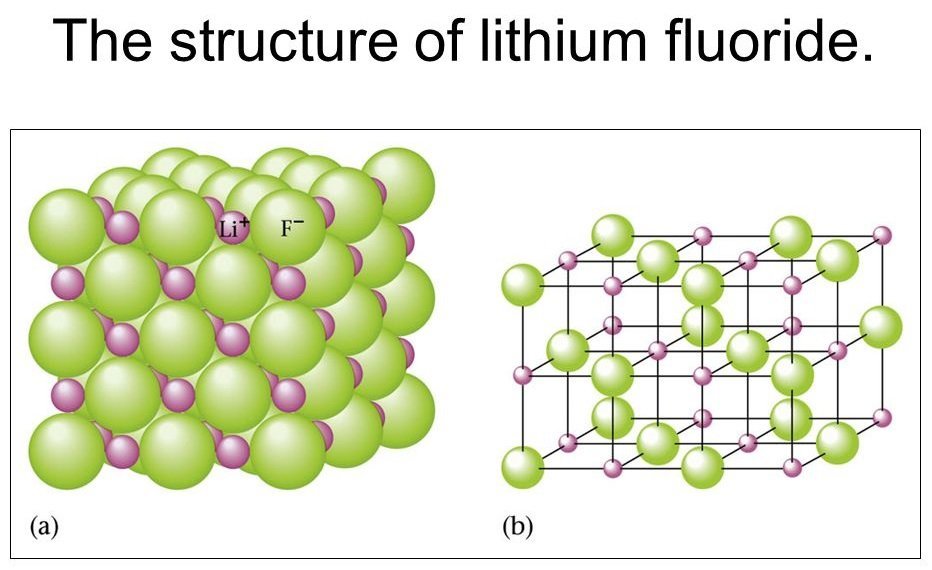
2. Natural Occurrence and Mineral Status
While LiF can occur naturally, it is not commonly found as a standalone mineral in significant quantities due to its low solubility and tendency to form in trace amounts within other lithium-bearing minerals.
- Geological environments:
- Pegmatites rich in lithium and fluorine
- Hydrothermal veins associated with granitic intrusions
- As an accessory mineral in lithium-rich micas like lepidolite
- Known occurrences:
- Some lithium pegmatite bodies in Canada, Brazil, and Madagascar
- Rare in metamorphic and volcanic environments

3. Synthetic vs. Natural Lithium Fluoride
While natural lithium fluoride is rare, synthetic LiF is widely used in various industries:
- Optical materials: Used in UV and IR windows and lenses due to its wide bandgap and transparency.
- Nuclear applications: Used in molten salt reactors and as a neutron-absorbing material.
- Electrolyte in lithium batteries: Used in specialized solid-state battery research.
- Flux in ceramics and glass: Helps lower melting temperatures during processing.

4. Comparison with Other Lithium Minerals
| Mineral | Chemical Formula | Lithium Content | Use |
|---|---|---|---|
| Spodumene | LiAlSi₂O₆ | High | Primary lithium source for batteries |
| Lepidolite | K(Li,Al)₃(Al,Si)₃O₁₀(F,OH)₂ | Moderate | Ceramics, minor lithium source |
| Petalite | LiAlSi₄O₁₀ | Moderate | Glass and ceramic production |
| Amblygonite | LiAl(PO₄)(F,OH) | Moderate | Ornamental use, minor lithium source |
| Lithium Fluoride | LiF | Low (by weight) | Industrial and scientific uses |
5. Industrial and Scientific Applications
Despite its rarity in nature, lithium fluoride is highly valued in technical applications:
A. Nuclear Industry
- Used in molten salt nuclear reactors due to its high thermal stability and compatibility with lithium and beryllium salts.
B. Optics and Electronics
- Employed in UV and infrared optics due to its wide transmission range.
- Used in scintillation detectors for radiation and particle physics.
C. Battery Research
- Being explored in solid-state batteries and lithium metal anode protection layers.
D. Metallurgy and Ceramics
- Acts as a flux in glass and ceramic manufacturing.
- Used in aluminum smelting to reduce energy consumption.
6. Safety and Handling
Lithium fluoride is not highly toxic, but it should be handled with care:
- Inhalation or ingestion of fine particles may cause irritation.
- Dust control is important in industrial environments.
- Storage: Keep in airtight containers away from moisture and reactive substances.

FAQs
Q1: Is lithium fluoride a naturally occurring mineral?
A1: Yes, but it is rarely found in significant quantities and is typically present as a trace component in lithium-rich pegmatites.
Q2: What is lithium fluoride used for?
A2: It is used in nuclear reactors, optical components, solid-state batteries, and as a flux in glass and ceramic production.
Q3: Is lithium fluoride toxic?
A3: It is low in toxicity but should be handled carefully to avoid inhalation or ingestion of dust.
Conclusion
Lithium fluoride (LiF) is a rare naturally occurring mineral with important industrial and scientific applications. While not a primary source of lithium for battery production, its thermal, optical, and nuclear properties make it a valuable compound in high-tech and research fields.

