Lithium Chloride Mineral
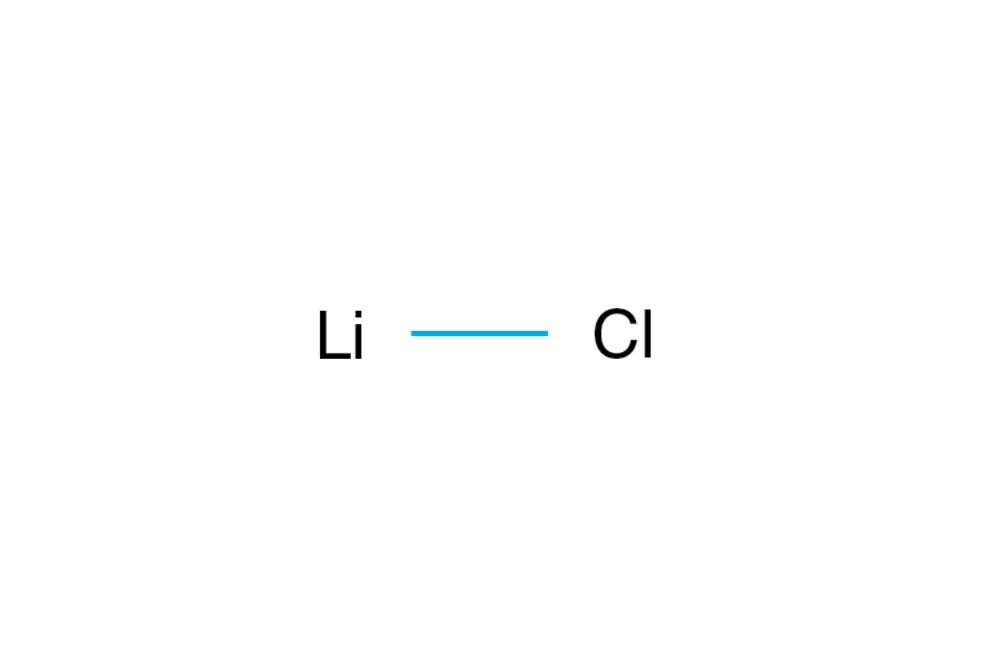
Lithium chloride (LiCl) is a chemical compound composed of lithium and chlorine. While it is not a mineral in its pure form, it can occur naturally in lithium-rich brines and is widely used in industrial and scientific applications.
Here’s a detailed look at lithium chloride, its properties, natural sources, and practical uses.
1. What Is Lithium Chloride?
Lithium chloride is an inorganic salt with the chemical formula LiCl. It is highly soluble in water, hygroscopic (absorbs moisture from the air), and has a high melting point.
- Chemical formula: LiCl
- Molar mass: 42.39 g/mol
- Appearance: White crystalline solid
- Melting point: ~610°C
- Solubility: Highly soluble in water and polar solvents
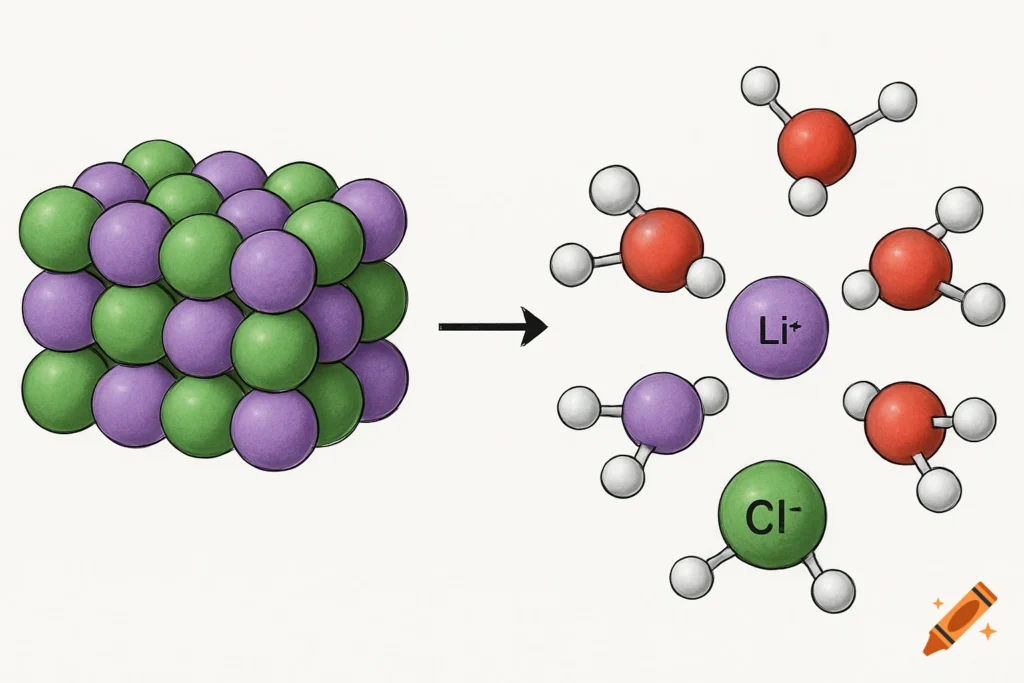
2. Natural Occurrence and Lithium Chloride Minerals
Lithium chloride is not commonly found as a standalone mineral in nature. However, it can be found in:
- Salt flats (salars): Especially in South America’s Lithium Triangle (Chile, Argentina, Bolivia), where lithium-rich brines contain dissolved lithium chloride.
- Geothermal brines: In regions with volcanic activity, lithium chloride can be extracted as a byproduct.
- Evaporite deposits: Formed from the evaporation of saline lakes and seas.
The mineral zabuyelite (Li₂CO₃) is a naturally occurring lithium carbonate, but lithium chloride is typically derived from processing lithium brines.
3. Industrial and Scientific Uses of Lithium Chloride
Lithium chloride is used in a variety of applications, including:
- Lithium metal production: Used as a precursor in the electrolytic production of metallic lithium.
- Desiccant: Due to its hygroscopic nature, LiCl is used in air drying and humidity control systems.
- Battery manufacturing: Used in the production of lithium salts for advanced battery electrolytes.
- Organic synthesis: Acts as a reagent in pharmaceutical and chemical synthesis.
- Research and nuclear applications: Used in molten salt reactors and neutron-absorbing materials.
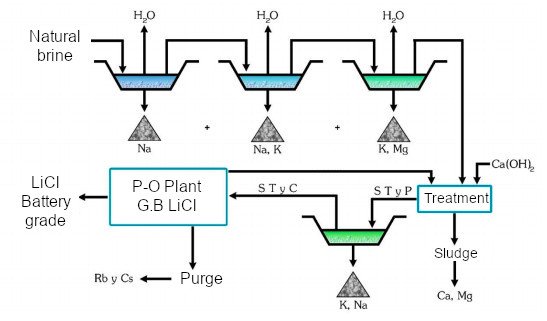
4. Extraction and Production
Lithium chloride is primarily obtained through:
- Solar evaporation: In brine operations, water is evaporated to concentrate lithium salts, including lithium chloride.
- Chemical precipitation: After concentration, lithium chloride is purified through chemical processes.
- Electrolysis: Used to produce metallic lithium from lithium chloride.
5. Safety and Environmental Considerations
While lithium chloride is not highly toxic, it should be handled with care:
- Irritant: Can cause irritation to skin and eyes.
- Environmental impact: Improper disposal can affect water systems and aquatic life.
- Storage: Should be kept in sealed containers to prevent moisture absorption.
Proper management of lithium chloride in industrial settings is essential for safety and environmental protection.

FAQs
Q1: Is lithium chloride a mineral?
A1: No, lithium chloride is a chemical compound, not a naturally occurring mineral. It is typically derived from lithium brines.
Q2: What is lithium chloride used for?
A2: It is used in lithium metal production, desiccants, battery electrolytes, chemical synthesis, and nuclear applications.
Q3: Where is lithium chloride found naturally?
A3: It occurs in lithium-rich brines, especially in salt flats like those in Chile and Argentina.
Conclusion
Lithium chloride is a key compound in the production of lithium-based materials and plays a crucial role in industries ranging from energy storage to pharmaceuticals. While not a mineral itself, it is closely linked to natural lithium brine resources and is essential for the global lithium supply chain.

