Lithium Carbonate Mineral
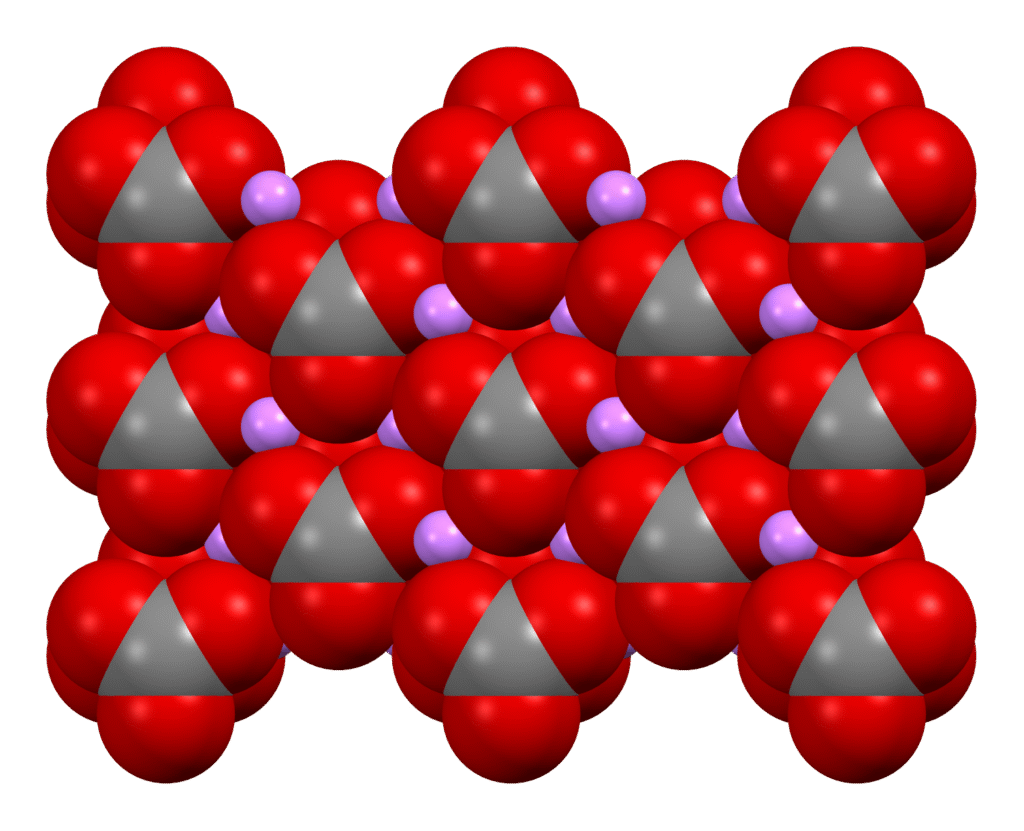
Lithium carbonate (Li₂CO₃) is a white, inorganic salt that plays a crucial role in both industrial applications and medicine. While it is not a naturally occurring mineral in its pure form, it can be derived from naturally occurring lithium-bearing minerals and brines.
Here’s a detailed overview of lithium carbonate, its properties, natural sources, and key applications.
1. What Is Lithium Carbonate?
Lithium carbonate is a chemical compound with the formula Li₂CO₃. It is the most commonly used lithium compound in industrial and pharmaceutical applications.
- Chemical formula: Li₂CO₃
- Appearance: White, odorless powder
- Solubility: Slightly soluble in water; solubility decreases with increasing temperature
- Melting point: ~723°C
- Primary use: Production of lithium-ion batteries and psychiatric medication

2. Natural Occurrence and Mineral Sources
While lithium carbonate is not found as a standalone mineral in nature, it can be derived from:
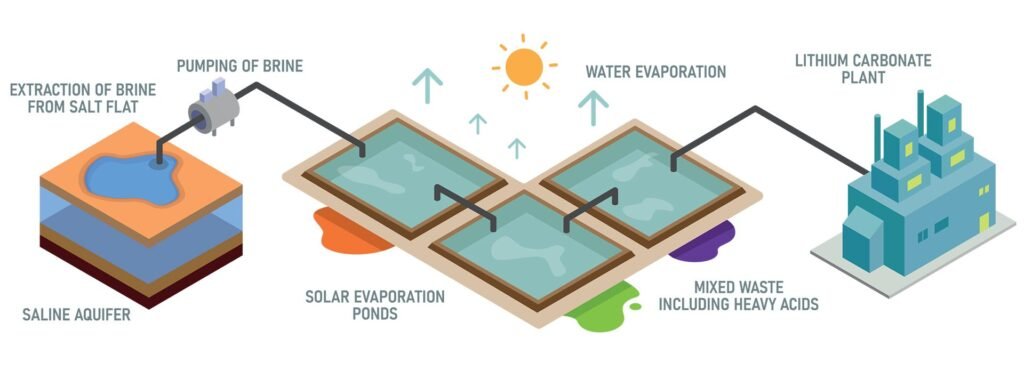
- Brine deposits: Lithium-rich brines in salt flats (e.g., Chile, Argentina) are evaporated to concentrate lithium salts, which are then processed into lithium carbonate.
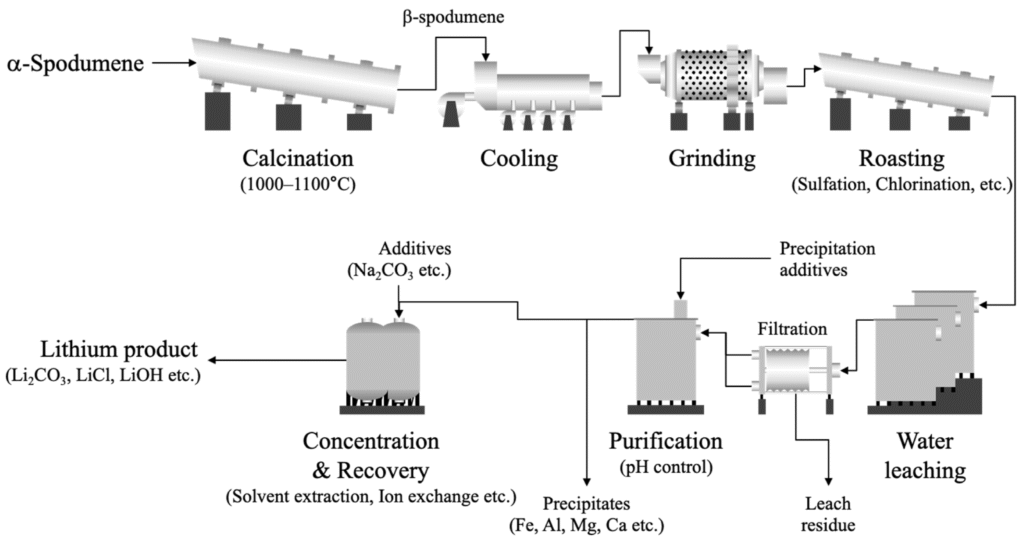
- Hard rock minerals: Lithium-bearing minerals like spodumene are processed to extract lithium compounds, including lithium carbonate.
- Geothermal brines: Emerging source where lithium is recovered as a byproduct of geothermal energy production.
A naturally occurring lithium carbonate mineral called zabuyelite (Li₂CO₃) has been identified in some geothermal environments, particularly in Tibet and parts of China.
3. Industrial and Medical Uses
Lithium carbonate is one of the most important lithium compounds, with two major applications:
1. Battery Production
Lithium carbonate is a key precursor in the production of lithium-ion batteries, used in:
- Electric vehicles (EVs)
- Smartphones and laptops
- Grid-scale energy storage systems
It is converted into other lithium compounds like lithium hydroxide for use in battery cathodes.
2. Psychiatric Medicine
Lithium carbonate is the most widely used form of lithium in medicine, particularly for treating:
- Bipolar disorder
- Mood stabilization
- Depression management
It works by modulating neurotransmitter activity and is considered a mood-stabilizing drug.
Image Description: Lithium-ion battery and a prescription bottle of lithium carbonate pills, showing its dual use in technology and medicine.
4. Extraction and Production Process
Lithium carbonate is typically produced through two main methods:
- From brines: Lithium-rich brines are pumped into evaporation ponds where water evaporates over several months, concentrating the lithium. The lithium is then reacted with sodium carbonate to form lithium carbonate precipitate.
- From hard rock: Spodumene is roasted and treated with sulfuric acid to produce lithium sulfate, which is further processed into lithium carbonate.
5. Environmental and Economic Considerations
As demand for lithium carbonate grows, so do concerns about:
- Water usage in brine extraction (especially in arid regions like Chile)
- Environmental impact of hard rock mining
- Recycling challenges for lithium-ion batteries
- Price volatility due to supply chain constraints
Efforts are underway to develop direct lithium extraction (DLE) technologies and battery recycling systems to improve sustainability.
FAQs
Q1: Is lithium carbonate a mineral?
A1: No, lithium carbonate is a chemical compound, not a naturally occurring mineral. However, a rare mineral form called zabuyelite exists in some geothermal regions.
Q2: What is lithium carbonate used for?
A2: It is primarily used in lithium-ion batteries and psychiatric medications for treating bipolar disorder.
Q3: How is lithium carbonate produced?
A3: It is extracted from lithium brines or derived from hard rock minerals like spodumene through chemical processing.
Conclusion
Lithium carbonate is a vital compound in both the technology and healthcare industries. As the demand for clean energy and mental health treatments continues to grow, sustainable production and ethical sourcing of lithium carbonate will be essential for the future of global industries.

